This week’s journal club focuses on a study from Cancer Chemother. Pharmacol. (2017) promoting Cantrixil as a promising anti-cancer agent targeting resilient cancer-stem–cell populations.
Saif et al. assess a new investigational compound, Cantrixil (TRX-E-002-1), for its anticancer potential, particularly targeting chemo-resistant cancer cells, and evaluate its pharmacology and toxicology in preclinical models. While many conventional chemotherapies fail against tumors with “stem-like”, slow-proliferating, drug-resistant sub-populations, Cantrixil is proposed to kill such cells, potentially preventing relapse.
To explore this, the researchers first demonstrated that Cantrixil has potent cytotoxic activity in vitro against a broad range of human cancer cell lines, including cancer-stem–cell–enriched lines (e.g., CD44+/MyD88+ ovarian cancer stem cells), with IC₅₀ values ≤ 0.1 µM in many cases. Then, in vivo studies in animal models, including disseminated ovarian cancer xenografts, recurrent ovarian cancer models, and orthotopic pancreatic cancer models, showed that intraperitoneal administration of Cantrixil significantly inhibited tumor growth and reduced tumor burden. For example, in the recurrent ovarian cancer mouse model, Cantrixil monotherapy decreased tumor burden by ~77%, and when combined with cisplatin, the effect was greater than either treatment alone.
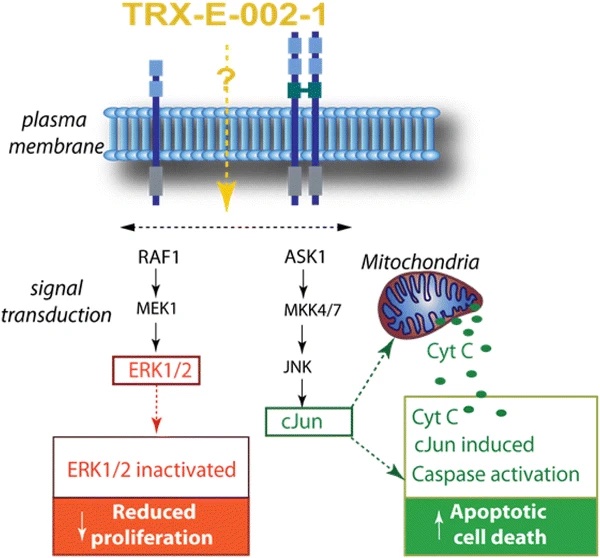
Mechanistically, Cantrixil induced caspase-mediated apoptosis in cancer cells, associated with increased phosphorylation of c-Jun and decreased phosphorylation of ERK, suggesting that Cantrixil shifts intracellular signaling to favor cell death over survival.
Importantly, before moving toward clinical trials, the authors evaluated the safety, pharmacokinetics, and genotoxicity of Cantrixil in GLP-compliant animal studies (rats and dogs). They conducted dose–tolerance, repeat-dose toxicity, ECG monitoring (for cardiac effects), mutagenicity assays (bacterial reverse mutation), and in vivo bone-marrow micronucleus assays to assess clastogenicity. They found that at clinically relevant doses, Cantrixil had an acceptable safety profile: no severe cardiac QT-interval prolongation in dogs, no mutagenesis in bacterial assays, and no significant chromosomal mutations in mammalian cells under typical exposure conditions.
However, at high doses (above maximum tolerated doses in rodents), toxic effects emerged, including testicular atrophy, lymphoid organ weight changes, and signs of gastrointestinal toxicity. The genotoxicity assay in mice did show an increase in micronucleated polychromatic erythrocytes at high doses, indicating potential clastogenic risk at those exposure levels.
Overall, the preclinical data suggest that Cantrixil has broad anticancer activity, including against chemo-resistant, stem-like cancer cells, and has a sufficiently favorable pharmacology and toxicology profile to warrant further development. The authors conclude that their results justify proceeding to a first-in-human clinical trial, likely via intraperitoneal administration in patients with refractory or recurrent ovarian (or other abdominal) cancers.
In sum: this study characterizes Cantrixil as a promising investigational anti-cancer agent that unlike many traditional chemotherapies, may target resilient cancer-stem–cell populations, potentially preventing relapse; and shows that, at therapeutic relevant doses, it does not raise prohibitive safety or genotoxicity concerns in preclinical models.
Journal Club: continue your reading here:
Saif MW, Heaton A, Lilischkis K, Garner J, Brown DM. Pharmacology and toxicology of the novel investigational agent Cantrixil (TRX-E-002-1).
Cancer Chemother Pharmacol. 2017 Feb;79(2):303-314. doi: 10.1007/s00280-016-3224-2
