Our research is focused on which cell fate mechanisms govern the type of regenerative strategy employed by a biological system.
We primarily use as model system the murine pancreas due to the wide-range of genetic tools available for this organ and its demonstrated cell plasticity potential. Besides employing classical systems of rapid cell-ablation (RIP-DTR, chemical ablation), we developed novel transgenic models of progressive β-cell decay (financed by Excellence

Map of pancreas plasticity (after Cigliola et al., 2016)
Project for Young Investigators of Novo Nordisk Foundation). By coupling the diverse β-cell ablation configurations with genetic cell tracing, timed conditional gene expression and diverse omics assays, we study the dynamical molecular fingerprint of β-cell death and the regenerative response employed, with focus on β-cell self-renewal potential (financed by FRIPRO Young Research Talent grant – Research Council of Norway). Moreover, we address the role of senescence-based cell-plasticity breaks on β-cell regeneration potential and modulate the age-related switch leading to β-cell quiescence by using genetic and pharmacological tools (financed by Excellence Project for Young Investigators of Novo Nordisk Foundation). Currently, we plan to activate the compensatory cell proliferation response in the β-cell compartment either by conditional gene expression or by releasing the proliferative instructive signals from β-cells trapped in a perpetual “undead” state.
Besides the in vivo approach, we also employ differentiating patient-specific induced pluripotent stem cells (iPSC) and organoids for disease modeling. We coupled these systems with large-scale imaging and omics in order to study β-cell fate acquisition, decay and proliferation (financed by STAMCELLER “STEMCELLS” Young Research Talent grant – Research Council of Norway). We recently characterized the positive impact of cell confinement on promoting the human iPSC differentiation potential towards the different pancreatic islet cell signatures, via an integrin-based mechanism (Legøy et al, 2020 Sci. Rep.). Currently we further investigate the influence of mechanical forces and adhesion on cell differentiation by using thermo-responsive hydrogels allowing the incremental alteration of their rigidity and density (financed by NCMM seeding grant).
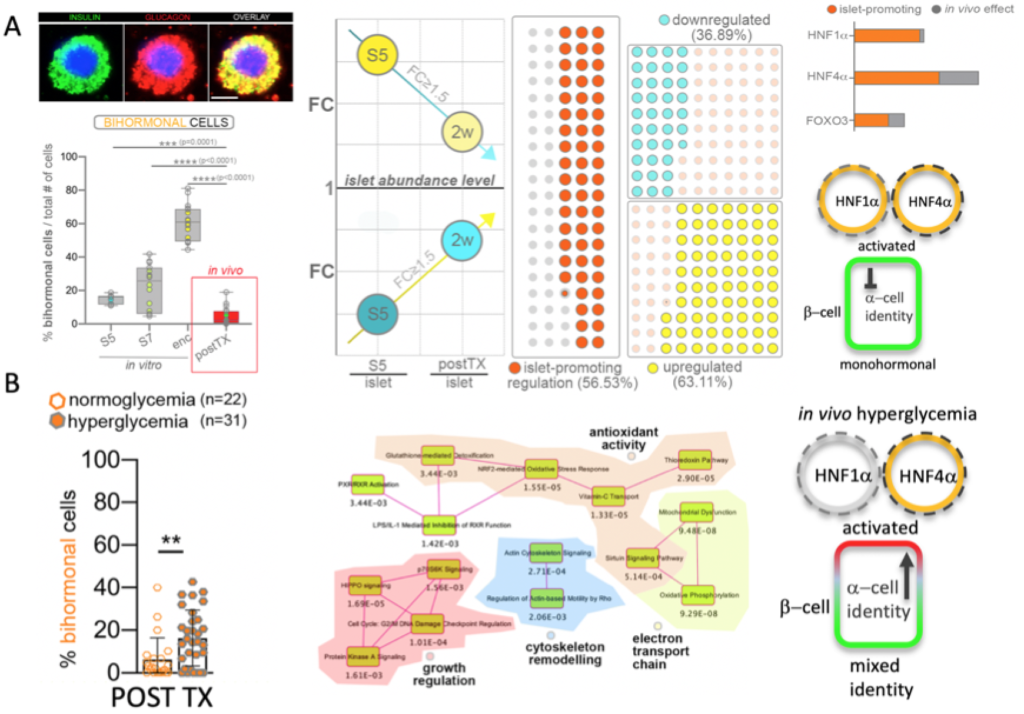
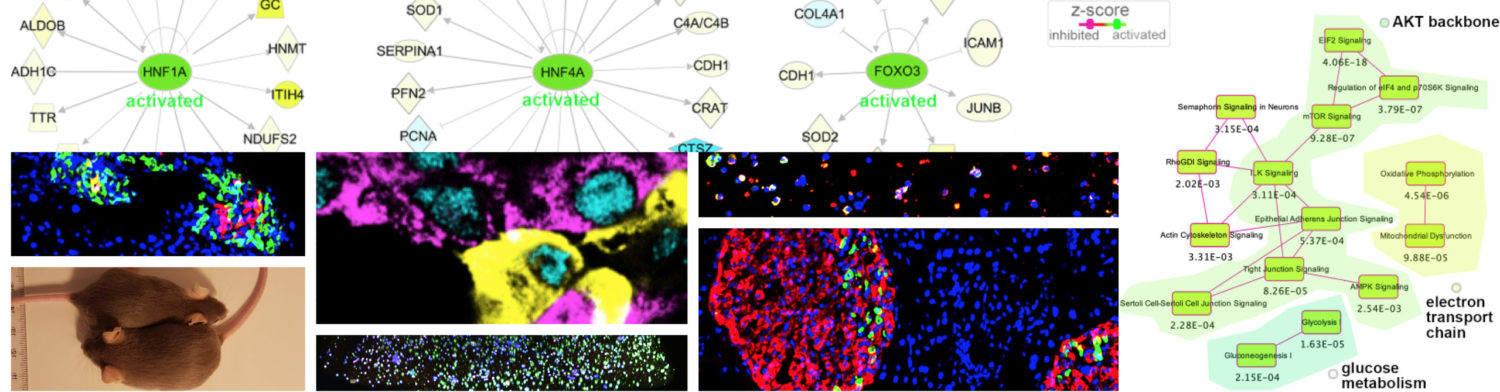
A. HNF1A and HNF4A are induced in vivo (from Legøy et al.,[Chera], Front Cell Dev Biol, 2020); B. hyperglycemia perturbs islet identity confinement (from Legøy et al.,[Chera] Acta Physiol, 2020).
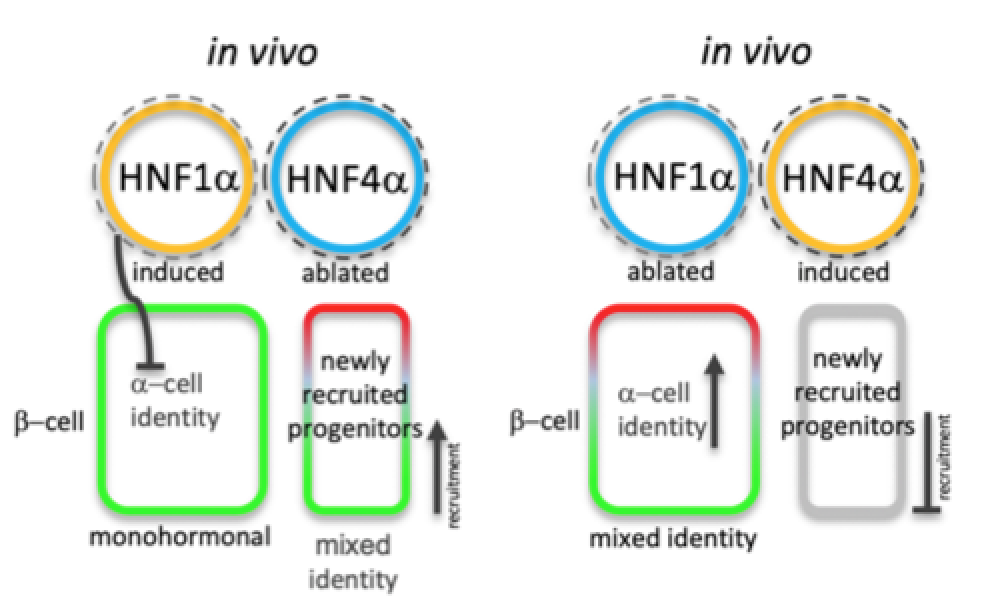
Schematic representation of the in vivo HNF1A / HNF4A balance tilt effects in humans (after Legøy et al.,[Chera] Front. Cell Dev. Biol. 2020).
Want to join the lab? Master and PhD student candidates interested in cell fate decisions, islet cell plasticity and molecular networks are encouraged to contact Simona Chera (simona.chera [at] uib.no).