A Short visit to the research: by Shayla Sharmine
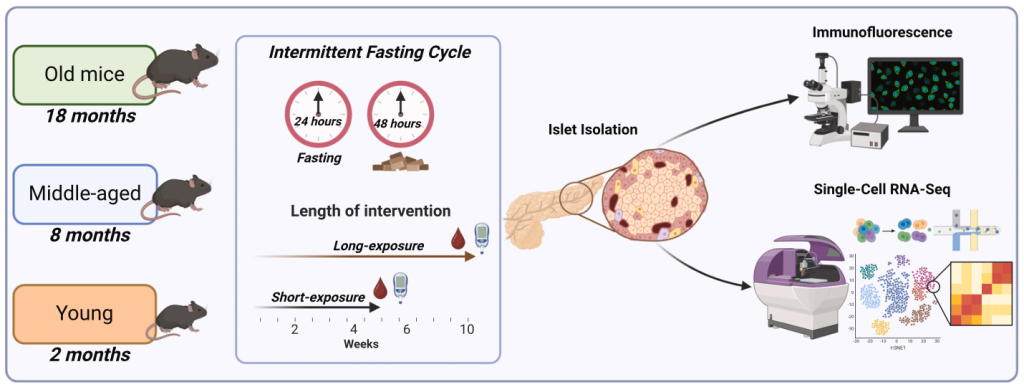
Intermittent fasting (IF) is a widely studied dietary intervention known for its metabolic benefits, including improved glucose homeostasis and insulin sensitivity. While IF has shown positive effects in adult and aging populations, its impact on younger individuals remains unclear. Adolescence is a critical period for pancreatic β cell development, making it essential to investigate whether fasting at this stage affects β cell maturation and function. A recent study published in Cell Reports on February 25, 2025, explored the age-dependent effects of short-term (ST) and long-term (LT) IF on glucose metabolism and pancreatic islet function in mice.
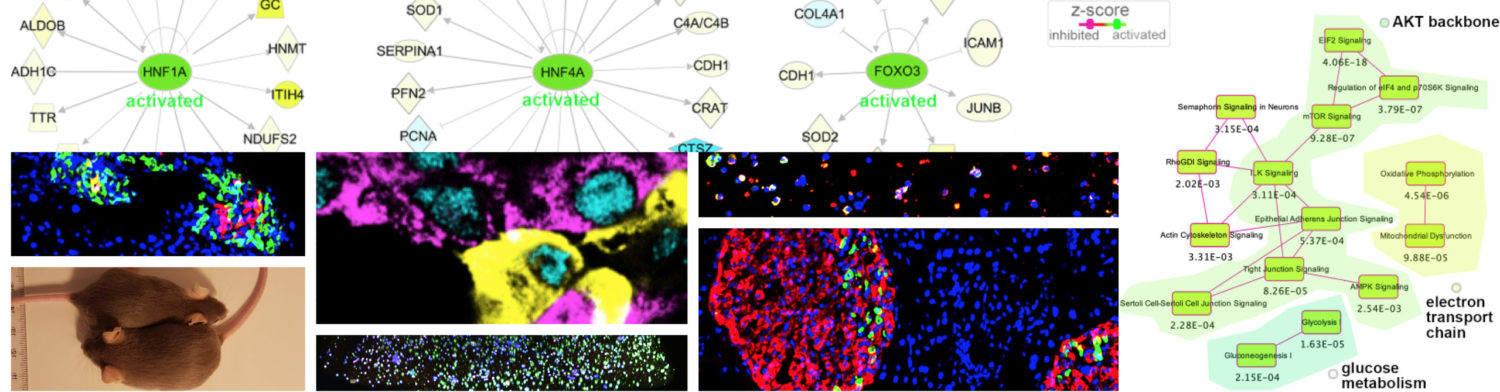
The study found that ST-IF improved glucose homeostasis across all age groups, whereas LT-IF had differential effects depending on age. Middle-aged and old mice experienced enhanced β cell function and insulin secretion, while adolescent mice exhibited impaired β cell maturation, reduced insulin secretion, and altered islet gene expression. Single-cell RNA sequencing (scRNA-seq) analysis revealed that young LT-IF mice had lower β cell maturation scores, decreased expression of key β cell genes (e.g., Mafa, Nkx6-1, Slc2a2, Ins1), and reduced insulin synthesis. These molecular alterations closely resembled transcriptional patterns observed in type 1 diabetes (T1D) patients.
Further analysis of human islet transcriptomic datasets confirmed that genes downregulated in IF-exposed young mice were similarly reduced in human T1D samples but not in type 2 diabetes (T2D). This suggests that extended fasting in adolescence could have unintended consequences on β cell function, potentially increasing susceptibility to β cell dysfunction.
Despite these findings, the study presents several challenges. The exact mechanisms underlying IF-induced β cell impairment in young mice remain unclear. Additionally, the study was conducted exclusively on male mice, leaving open questions about sex-specific differences in metabolic responses. Since different IF protocols may have varying effects, there is a window to explore comparative fasting regimens, such as time-restricted feeding or alternate-day fasting. While mice studies are not entirely relatable to humans, These findings highlight the need for further dissections in younger populations.
Beyond general metabolic health, there is significant scope for exploring the impact of IF on monogenic forms of diabetes, such as MODY (Maturity-Onset Diabetes of the Young), where single-gene mutations impair β cell function. Since IF influences β cell stress pathways, insulin production, and gene regulation, studying its effects in MODY subtypes (e.g., HNF1A-MODY, GCK-MODY) could reveal whether fasting exacerbates or alleviates β cell dysfunction in these conditions. Understanding how fasting interacts with monogenic diabetes pathways may provide new therapeutic insights and help personalize IF-based dietary recommendations for individuals with genetic predispositions to diabetes.
Continue your reading here:
Matta L, Weber P, Erener S, Walth-Hummel A, Hass D, Bühler LK, Klepac K, Szendroedi J, Guerra J, Rohm M, Sterr M, Lickert H, Bartelt A, Herzig S. Chronic intermittent fasting impairs β cell maturation and function in adolescent mice. Cell Reports 2025 Jan 18;44(2):115225. doi: 10.1016/j.celrep.2024.115225