by Shayla Sharmine
The endocrine pancreas produces hormones that regulate glucose homeostasis in the body. Among three major regulatory hormone-producing cell types, delta cell is known to regulate the secretion of somatostatin that counter-regulates insulin and glucagon production through intra-islet cross-talking. Recent studies have focused on the secretion pattern, alteration, and mechanism behind alteration and modulation due to the pathophysiological condition of somatostatin-producing delta cells. A new study published in Acta Physiologica earlier this year focused on monitoring somatostatin secretion from isolated single islets by developing a novel reporter-mediated cell assay. The finding shows somatostatin is hypersecreted from single islets of T2D donors, reconfirming previous findings of somatostatin dysfunction in T2D diabetes and establishing reporter cell real-time monitoring of somatostatin as a detection method. Somatostatin level measurement has been a research bottleneck due to the rapid degradation rate and lack of availability of immunoassay with higher sensitivity. This method poses an alternative to such limitations.
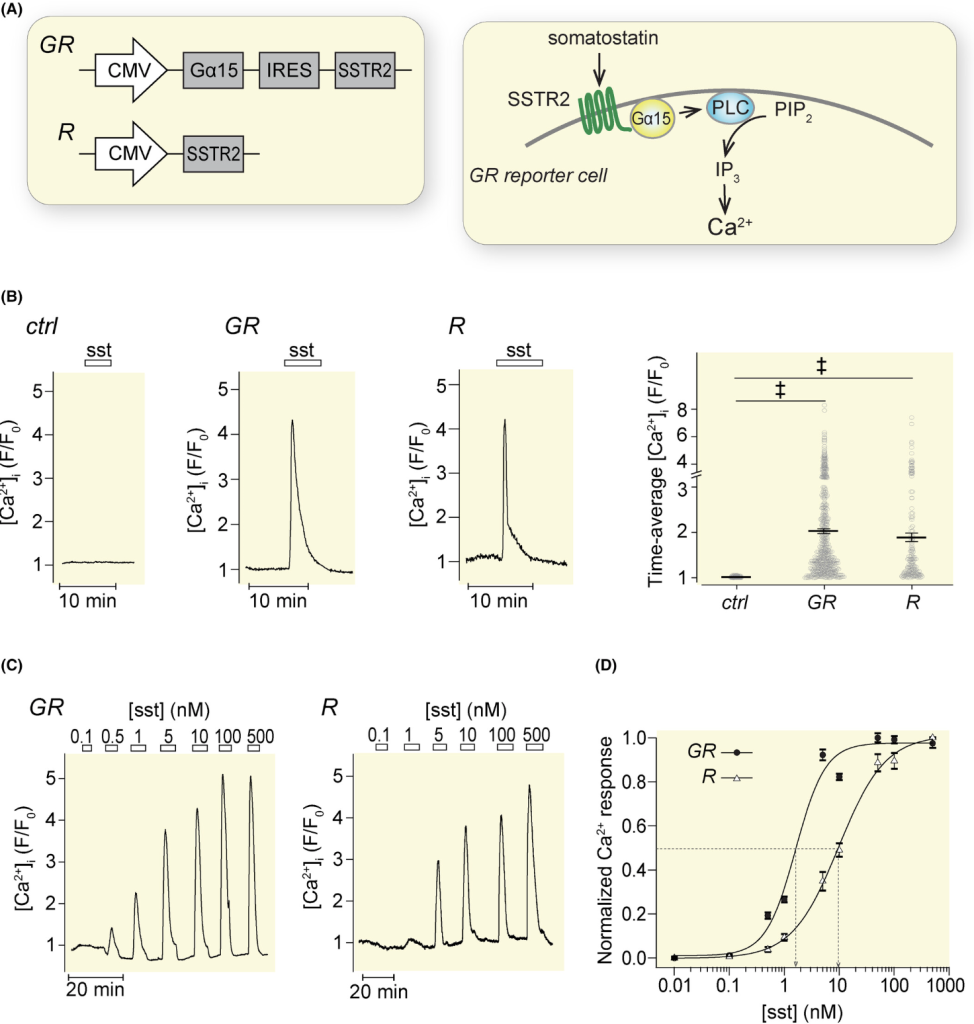
Here, Somatostatin receptors SSTR2 and Gα15 were expressed in HeLa cells, linking somatostatin binding to detectable calcium signaling. These GR-reporter cells responded to somatostatin in a dose-dependent manner and were used to monitor hormone release from both mouse and human islets. Stimulation by glucose, K⁺-induced depolarization, and hormones such as GLP-1, glucagon, and ghrelin evoked somatostatin release detected by the reporter system. The assay enabled synchronized recordings of calcium dynamics in islets and reporter cells. The method provides semi-quantitative, high-resolution kinetic data from single islets in real-time.
Experiments using human islets from T2D donors exhibited somatostatin hypersecretion in response to glucose and K+depolarization despite having fewer δ-cells under confocal microscopy. Single-islet measurements showed that this heightened secretion occurred under high glucose and K+ stimulation, while reduced secretion is observed in response to basal stimulation. These findings suggest that excessive somatostatin release in T2D may be a compensatory mechanism that might have a role in exacerbating dysregulation of impaired glucose homeostasis.
This technology opens new opportunities to investigate targeted interventions that modulate δ-cell activity to improve islet hormone balance in diabetes.
Continue your reading here:
Yang, M., et al. (2025). “Real-time detection of somatostatin release from single islets reveals hypersecretion in type 2 diabetes.” Acta Physiologica 241(2): e14268. doi: 10.1111/apha.14268