This week’s journal club focuses on a study from Frontiers in Bioengineering and Biotechnology (2023) aiming to assess the effects of a microencapsulation system based on the use of “human elastin-like recombinamers” (ELRs) on SC-islets.
by Agnes Sandvik
Aim
In the introduction they point to the fact that therapeutic applications for T1D could benefit from using hiPSC-derived islets as this could function as a potential cure, as the loss of these islets is what causes T1D. Their aim is to figure out how to provide immunoprotection for the islets so they won’t be rejected by the host, without having to put the host on immunosuppression drugs. They already did this in a different trial where they used sodium alginate-based microcapsules, but they still want to look into new methods to be able to transplant larger volumes of tissue, as the sodium alginate capsules only has a diameter of about 500 µm.
Methods
In this trial they wanted to test using human elastin-like recombinamers (ELRs) as a microcapsule for b-cell like spheroids. ELRs are artificial proteins made recombinantly in bacteria to mimic the elastin of the extracellular matrix, making them very biocompatible (the immune system recognizes them as ‘self’) and allowing influx of oxygen and glucose, as well as outflux of insulin as they are semipermeable.
They purchased the hiPSC from Alstem Inc, then first made hiPSC spheroids and further differentiated them into b-cell like spheroids. They refer to published methods for both steps. They also got the ELRs that they used for encapsulation from a professor in Spain and they put about 1000 cell spheres in a cell strainer to remove any liquid and dipped them alternately in two different ELRs (VKV-cyclo and RGD-N3) to create 6 bilayers that react with each other to form a hydrogel coating around the spheroids through a reaction called click-chemistry before transplanting the spheroids into mice.
Results
In vitro function of differentiated hiPSC
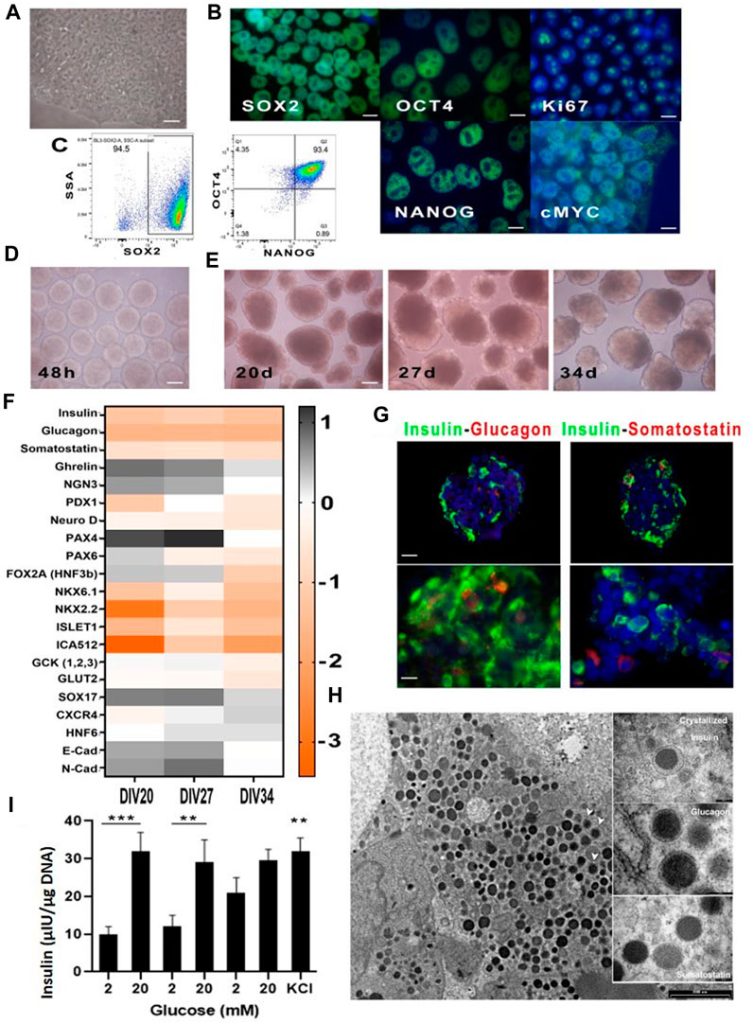
The cells grew well with identical morphology and the immunofluorescence showed pluripotency as the cells were positive for Oct4, Sox2, Nanog, c-Myc (genes that keep cells in a stem-like undifferentiated state) and Ki67 (proliferation marker). They also did flow cytometry and found that > 90 % of the cells were positive for Sox2, Oct4 and Nanog at the same time showing they are highly homogenous. (Fig1)
They also said that their selected differentiation protocol worked and in fig 1E they show images of the aggregates after 48h (just before starting the differentiation) at 20 days (just before starting day 6/terminal differentiation) and then at day 27 and 34 (two different end points they chose for the protocol). The aggregates increased in diameter and were no longer homogenous, showing cell maturation and structural changes.
Fig 1F q-pcr for many factors involved in the differentiation and to confirm that key pancreatic transcription factors were expressed (PDX-1, NKX6.1, SOX9, PTF1a, FOXA2, SOX17, CSCR4, c-KIT, Glut2 and Glucokinase) as well as mature ϐ-cell markers: MafA, MafB, NKX2.2, NKX6.1. They found that most of the spheroids had expected changes in genetic expression patterns.
They also checked the 27 day spheroids by immunofluorescence for insulin(b-cells), glucagon(α-cells) and somatostatin(δ-cells) and found strong insulin signals and weaker signals for the latter, indicating that most of the cells had a b-like characteristic. TEM (transmission electron microscopy) also showed insulin granules inside the spheroids, this was both mature(crystallized) and newly synthesized insulin.
There were however some cell aggregates that didn’t show any granule content, they had likely followed a different differentiation path, they suggested liver as these two organs have common developmental origins.
When they chose the aggregates that were correctly differentiated and incubated them with glucose they saw that they were able to respond physiologically to shifting glucose concentration. The spheroids secreted less insulin than real human islets, but the kinetics were ultimately the same. Finally when they stimulated insulin release at the end of this test using KCl it was confirmed that the b-like cells also were able to store insulin.
The kinetics is again supported by TEM that clearly shows insulin containing cytoplasmic granules.
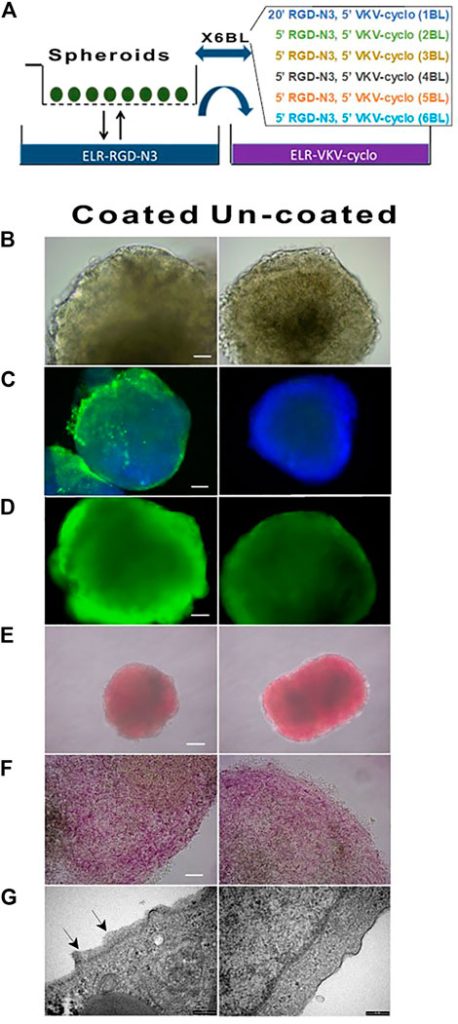
ELRs coating of cell spheroids
They performed this alternating coating with the two ELRs (RGD-N3 and VKV-Cyclo) as sown in 2a. After the coating they didn’t see any change in morphology (2b), viability (2d) or hormone content (2e,f). They also state that they didn’t see difference in mRNA levels or functional performance, but this data is not shown. In 2c they stained with an anti-elastin antibody to show the presence of the coating.
They analyzed the robustness of the coating itself by keeping the spheroids in culture, now without rotating to protect the coating, and it stayed intact. It also survived shipment and handling, but during early polymerization it was very vulnerable so temperature control during coating was crucial.
They stained again with DTZ, binding to Zn in insulin to stain it in red and saw that the spheroids intensely and uniformely stained red, confirming the content of processed insulin. (2E shows intact- red stained spheroids, 2f shows the same spheroids crushed under a coverslip, releasing red-stained insulin-containing cells.)
They also studies the coating thickness using TEM, confirming a thin surface layer (20-200nm) on the spheroids, while the uncoated spheroids lacked this layer, directly proving the layers existence.
Identification of cell markers
In both their immuno and qPCR analysis they included UNC3 as it is expressed in late stages of b/α-cell differentiation and increases at differentiation to mature endocrine cells. In fig 3 they compare differentiated cell aggregates with real human islets both through staining and qPCR.
They stained for UCN3 within insulin and glucagon positive cells and the UCN3-exprssing cells had a merging color between red and green which they think suggests (but doesn’t prove) insulin-glucagon co-localization
In the qPCR results the black and white histograms were used to separate the optimally and sub-optimally differentiated aggregates.
Optimal:
- UCN3 mRNA levels increased together with insulin mRNA and the expression pattern seemed very similar to human islets, but they still had 15x less insulin mRNA than human islets and much lower glucagon and somatostatin expression.
Sub-optimal: two examples:
- Div27-G: produces a lot of glucagon but little insulin, with somatostatin almost undetectable (α-cell like identity).
- Div27-S: made somatostatin, but not insulin or glucagon (indicated δ-cell like identity). They all still express UCN3 and HLA-G5, indicating that these markers not only indicate b-cells but rather maturity within any endocrine lineage.
In vivo studies:
They transplanted the differentiated spheroids into immune-incompetent NOD/SCID mice to study both if they could survive in vivo and also to figure out where the most suitable graft site was.
They first tried to transplant both coated and uncoated spheroids into the epididymal fat pad and intramuscular. But these could not be recovered, indicating that they didn’t survive or integrated poorly in these locations.
They then tried transplantation into the peritoneal cavity (space inside the abdomen surrounding the organs), which turned out to be the only location where the spheroids survived long enough to be recovered.
When they recovered the spheroids they found that both coated and uncoated spheroids did survive, but the uncoated had dead areas and were less structurally intact, proving that ELR coating helped to protect the cells in vivo. The coating was still present when they studied the spheroids under a microscope, but not perfectly uniform.
Assessment of in vivo survival in CD1 mice
Finally, they tested whether the ELR coating provided immune protection by transplanting them into immunocompetent mice (that would normally reject human cells).
About 1000 spheroids were transplanted into the peritoneal cavity of the mice and samples were collected at a short term (24-48hours) and long term (up to 42 days) after transplantation. After 48 hours the uncoated spheroids triggered an immune response and when they examined the tissue through histology they found very few surviving uncoated spheroids. For the ELR coated spheroids, they could be retrieved at all scheduled times (48h, 7days, 21days, 42days) and microscopy showed that the cells inside the coated spheroids kept their morphology and viability and that the amount of produced insulin were either increased or consistent with the time of transplantation.
Lastly they also examined the mouse’s peritoneal cavity after the experiment was done and they did not find any visible inflammation/infection.
Conclusions
They conclude that the ELRs coating did not interfere with any biological properties or the viability of the differentiated hiPSCs and that it seems to offer immunoprotection to the cells in preliminary in vivo studies, so they can’t make a definite conclusion of this yet, but it seems promising. When it comes to the question if these b-cell like spheroids actually correct for hyperglycemia they are still testing for this so they can’t say anything about it yet.
Continue your reading here:
Montanucci P, Pescara T, Greco A, Basta G, Calafiore R. Human induced pluripotent stem cells (hiPSC), enveloped in elastin-like recombinamers for cell therapy of type 1 diabetes mellitus (T1D): preliminary data.
Frontiers in Bioengineering and Biotechnology Sec. Tissue Engineering and Regenerative Medicine 2023 Apr 25;11:1046206. doi: 10.3389/fbioe.2023.1046206

1 comment for “Journal Club: Human induced pluripotent stem cells (hiPSC), enveloped in elastin-like recombinamers for cell therapy of type 1 diabetes mellitus (T1D)”