This week’s journal club focuses on a study from non-coding RNA (2025) aiming to to investigate the roles of the mouse homolog of Hnf1aos1 in liver function, gene expression, and cellular processes.
by Shayla Sharmine
The liver is the primary regulatory organ for maintaining metabolic homeostasis, detoxification, and immune response. A key challenge in maintaining homeostasis arises from disrupted external and internal physiological challenges, primarily centered on but not limited to diet, pancreatic dysfunction, inflammation, and chronic liver diseases. Several studies unraveled the emerging role of Long non-coding RNAs (lncRNAs) as key players in terms of tissue and organ development, homeostasis, as well as disease progression. While several lncRNAs have already been recognized in liver disease, this only opens the doors to continue deeper investigation, exploring roles by using animal and in vitro models. In human Hepatocyte Nuclear Factor 1 (HNF1A) and its antisense lncRNA, HNF1A AS1 acts as a regulatory pair in liver biology. The mouse ortholog of human HNF1A-AS1, HNF1A-aos1, was utilized in the current study.
The study was designed with a crucial aim in mind: to investigate the role of the mouse long non-coding RNA, HNF1aos1, in liver function, gene expression, and cellular processes in mice.
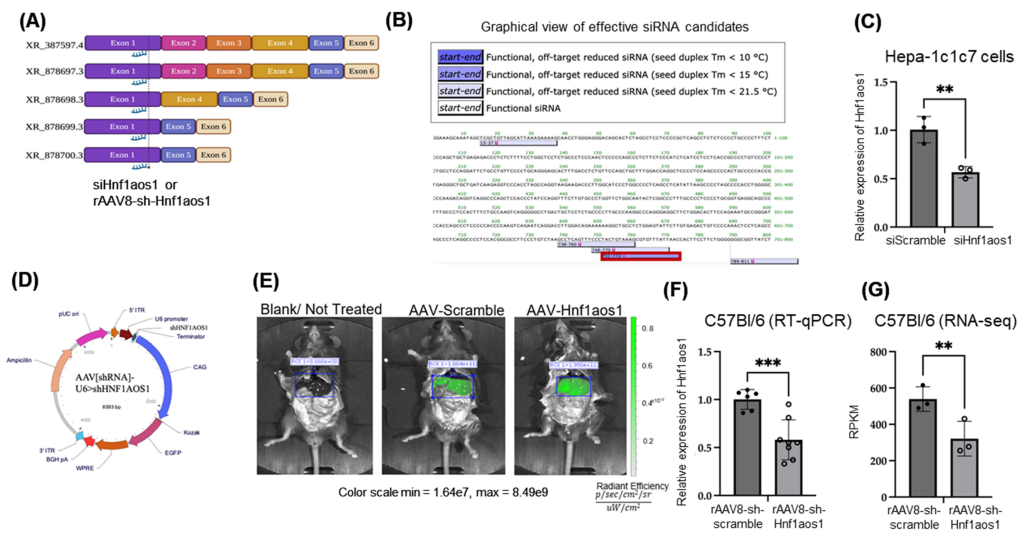
To achieve the aim, knockdown of HNf1aos1 was performed using AAV-shRNA complexes that targeted the liver. HNF1aos1 expression was suppressed in the mouse liver cell line using siRNA. RNA-seq and proteomics analysis were performed to reveal global changes in transcriptome and protein expression levels, following RT-qPCR to verify the expression of selected genes. Liver tissue histology and physical assessment were performed to visualize morphological and functional changes.
The results suggested a broad spectrum of expression profile change of the key players of lipid metabolism, hepatic inflammation, and compensatory acute phase signaling. Among these, the most interesting and unconventional finding involved reduced hepatic triglyceride levels, while an increased hepatic steatosis pattern was observed. The RNA seq analysis suggested suppression of de novo lipogenesis through downregulation of 2 key genes, SREBF1 and SCD1. Downregulation of very low-density lipoprotein receptor (VLDLR) in the liver was suggestive of the liver’s reduced ability to process circulating lipids. The authors mentioned it as a paradoxical outcome consistent with advanced metabolic steatotic liver disease, where lipotoxicity and impaired lipid metabolism trigger liver damage, followed by increased hepatic inflammation and disrupted liver architecture. This was correlated to fatty liver from mice treated with High Fat Diet ( HFD), showing significantly lower expression of HNF1aos1 compared to the control group, representing natural suppression of HNF1aos1 when challenged with diet. These findings have significant implications for understanding the role of HNF1aos1 in liver function and lipid metabolism.
To conclude, this study explored the role of lncRNA HNF1aos1 in maintaining hepatic lipid homeostasis, modulating lipid-induced inflammatory responses, and antiviral defense. There are several scopes in the current study, which remained unexplored, for example, how the level of HNF1A was impacted, and the level of TG in the HFD-treated knocked down mice. Regardless, this definitely opened up the possibilities of future investigation that can be performed in the context of diseases like obesity, obesity induced insulin resistance, and hyperglycemia.
Continue your reading here:
Armanios, B., Jin, J., Kolmel, H., Laddha, A. P., Mishra, N., Manautou, J. E., & Zhong, X.-B. (2025). Unraveling the Regulatory Impact of LncRNA Hnf1aos1 on Hepatic Homeostasis in Mice. Non-Coding RNA, 11(4), 52. Retrieved from https://www.mdpi.com/2311-553X/11/4/52
