 In 2008 I seized the chance of working on murine regeneration mechanisms in Prof. Pedro L. Herrera group. This was indeed an outstanding opportunity due to the wide range of powerful tools available in mice, however missing from most other regenerative systems. Moreover, the scarcity of the mammalian regeneration systems, rooted in their poor regenerative potential, makes Prof. Herrera’s highly controllable setup of insulin-producing β-cell genetic ablation and regeneration one of the best murine regenerative systems to date. Upon joining his group, I further pursued my interests in the characterization of the cellular processes and molecular cues governing the balance between tissue regeneration and homeostasis with emphasis on pancreatic β-cell regeneration and general cell plasticity leading to islet cell type interconversion phenomena. This line of research culminated with the characterization of two age-dependent regenerative mechanisms involved in spontaneous murine β-cell regeneration (Thorel et al. 2010 Nature and Chera et al. 2014 Nature). The study on the age-related differences was initiated based on my special interest in studying the influence of aging on regenerative processes. The outcome was beyond expectation, showing that very young mice can all spontaneously and naturally recover from diabetes extremely efficient, by a completely different cellular and molecular mechanism of β-cell regeneration than in their adulthood. This mechanism involves the dedifferentiation, proliferation and asymmetric re-differentiation of a different pancreatic hormonal cell type.
In 2008 I seized the chance of working on murine regeneration mechanisms in Prof. Pedro L. Herrera group. This was indeed an outstanding opportunity due to the wide range of powerful tools available in mice, however missing from most other regenerative systems. Moreover, the scarcity of the mammalian regeneration systems, rooted in their poor regenerative potential, makes Prof. Herrera’s highly controllable setup of insulin-producing β-cell genetic ablation and regeneration one of the best murine regenerative systems to date. Upon joining his group, I further pursued my interests in the characterization of the cellular processes and molecular cues governing the balance between tissue regeneration and homeostasis with emphasis on pancreatic β-cell regeneration and general cell plasticity leading to islet cell type interconversion phenomena. This line of research culminated with the characterization of two age-dependent regenerative mechanisms involved in spontaneous murine β-cell regeneration (Thorel et al. 2010 Nature and Chera et al. 2014 Nature). The study on the age-related differences was initiated based on my special interest in studying the influence of aging on regenerative processes. The outcome was beyond expectation, showing that very young mice can all spontaneously and naturally recover from diabetes extremely efficient, by a completely different cellular and molecular mechanism of β-cell regeneration than in their adulthood. This mechanism involves the dedifferentiation, proliferation and asymmetric re-differentiation of a different pancreatic hormonal cell type.
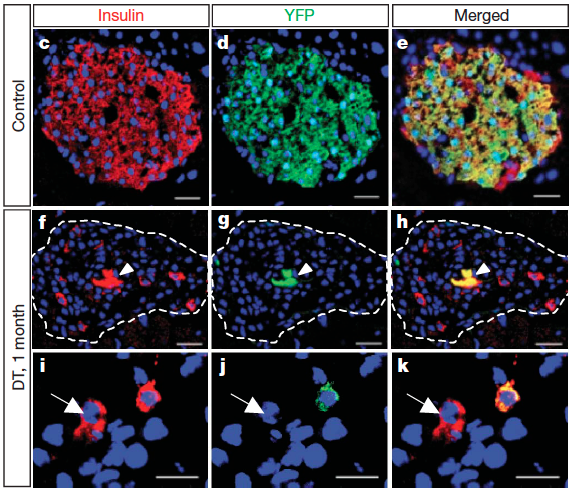
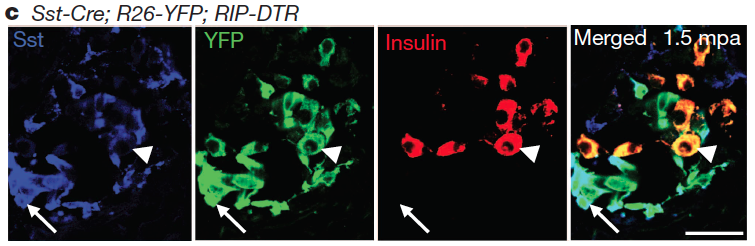 These studies required a substantial amount of mouse genetics and, to be able to perform in vivo the required inducible cell-tracing experiments, targeted cell-ablation, and inactivation of target-genes, I successfully helped conceive and employ more than 12 different multiple transgenic setups, some bearing up to 7 transgenes. By using these setups I was able to genetically trace 7 different cell populations, ablate the three main hormonal cell types of the pancreatic islet and transiently inhibit the transcription of a specific gene.
These studies required a substantial amount of mouse genetics and, to be able to perform in vivo the required inducible cell-tracing experiments, targeted cell-ablation, and inactivation of target-genes, I successfully helped conceive and employ more than 12 different multiple transgenic setups, some bearing up to 7 transgenes. By using these setups I was able to genetically trace 7 different cell populations, ablate the three main hormonal cell types of the pancreatic islet and transiently inhibit the transcription of a specific gene.
Besides mouse genetics, I enriched my set of skills by participating in several highly specialized courses concerning the analysis of high-throughput data and high performance computing. As result, I performed and analyzed the dynamic molecular fingerprint characterizing the timeline of regenerative events by employing several gene-expression (RNA-seq) and miRNA profiles (submitted manuscript).

Complete publication list related to my postdoctoral work:
- Cigliola V, Ghila L, Thorel F, van Gurp L, Baronnier D, Gupta S, Miyatsuka T, Kaneto H, Magnuson MA, Osipovich AB, Sander M, Wright C, Thomas MK, Furuyama K, Chera S and Herrera PL, Pancreatic Islet-Autonomous Signals Modulate Identity Changes of Glucagon+ α-Cells, Nature Cell Biology 2018 Nov;20(11):1267-1277. doi: 10.1038/s41556-018-0216-y. PMID: 30361701
- Chakravarthy H, Gu X, Enge M, Dai X, Wang Y, Damond N, Downie C, Liu K, Wang J, Xing Y, Chera S, Thorel F, Quake S, Oberholzer J, MacDonald PE, Herrera PL, Kim SK. Converting Adult Pancreatic Islet α Cells into β Cells by Targeting Both Dnmt1 and Arx, 2017 Cell Metabolism 25(3):622-634. doi: 10.1016/j.cmet.2017.01.009 PMID:28215845
- Chera S, Herrera PL. Regeneration of Pancreatic Insulin-Producing Cells by In Situ Adaptive Cell Conversion, 2016 Current Opinion in Genetics and Development 40:1-10, PMID: 27266969
- Cigliola V, Thorel F, Chera S, Herrera PL. Stress-Induced Islet Cell Identity Changes, 2016 Diabetes, Obesity and Metabolism 18(Suppl 1):87-96. doi: 10.1111/dom.12726 PMID: 27615136
- Chera S, Baronnier-Caffé D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, Herrera PL. Diabetes recovery by age-dependent conversion of pancreatic δ- or α-cells into insulin producers 2014 Nature 514(7523):503-507. PMID: 25141178 Comment in Diabetes: reprogrammed pancreatic δ-cells restore insulin production. [Nat Rev Endocrinol. 2014]
- Fujikawa T, Berglund ED, Patel VR, Ramadori G, Vianna CR, Vong L, Thorel F, Chera S, Herrera PL, Lowell BB, Elmquist JK, Baldi P, Coppari R. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin 2013 Cell Metab. 18(3):431-44. PMID: 24011077 Comment in Leptin, GABA, and glucose control. [Cell Metab. 2013]
- Thorel F, Damond N, Chera S, Wiederkehr A, Thorens B, Meda P, Wollheim CB, Herrera PL. Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice 2011 Diabetes 60(11):2872-82. PMID: 21926270
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of Adult Pancreatic a-cells to b-cells After Extreme b-cell Loss 2010 Nature 464(7292):1149-54. PMID: 20364121 Comments in: Diabetes forum: Extreme makeover of pancreatic alpha-cells. [Nature. 2010] Differentiation: from alpha to beta. [Nat Rev Endocrinol. 2010]