by Ulrik Larsen
This study uncovered a new amygdala–hypothalamus–liver axis that controls how the body adapts metabolically to acute stress (the fight-or-flight response), independent of the classical HPA stress pathway. Importantly, this circuit could be triggered without fear as activation of the amygdala was enough to change the metabolism.
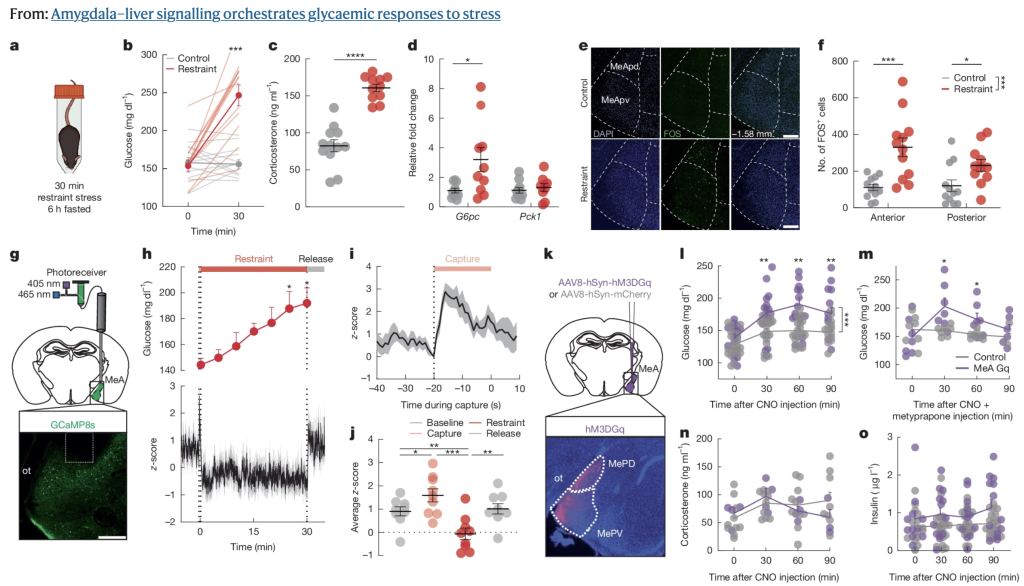
Using different stressors on mice, the group pinpointed the medial amygdala (MeA) as the key site activated during acute stress. The MeA is specialized for detecting innate threats (sight, smell, social), as opposed to learned or chronic stress. From there, MeA neurons send signals to the ventromedial hypothalamus (VMH), which directly influences the liver via the sympathetic nervous system. This provides a fast-track metabolic response without waiting for slower hormonal systems (like glucocorticoids or glucagon).
When the researchers used chemogenetics (DREADD) to artificially activate MeA neurons, mice showed higher blood glucose and reduced feeding — but no changes in anxiety or fear behavior.
They then proposed that the brain has two parallel threat-response circuits:
- CeA (learned fear)/BNST (chronic fear) → HPA/autonomic centers → fear behaviors + sustained cortisol release.
- MeA (innate fear) → VMH → liver → rapid glucose release + feeding suppression.
(There is a lot of overlap between the pathways, so one mechanism can induce both).
Potential implications:
- For animal studies: if you want accurate measures of glycemia or food intake, it may help to habituate mice to handling beforehand, so they don’t trigger the MeA pathway from new stress.
- For human health: repeated or prolonged stress could dysregulate this MeA circuit, potentially explaining why chronic stress contributes to metabolic disorders like type 2 diabetes.
Main message
Stress metabolism can be uncoupled from fear — meaning the body might “gear up” for survival even when we don’t consciously feel afraid. Innate fears such as sight, smell and social stress were shown to activate MeA neurons
Continue your reading here:
J. R. E. Carty, K. Devarakonda, R. M. O’Connor, A. Krek, D. Espinoza, M. Jimenez-Gonzalez, A. Alvarsson, R. F. Hampton, R. Li, Y. Qiu, S. Petri, A. Shtekler, A. Rajbhandari, K. Conner, M. Bayne, D. Garibay, J. Martin, V. Lehmann, L. Wang, K. Beaumont, I. Kurland, G. C. Yuan, P. J. Kenny & S. A. Stanley. Amygdala–liver signalling orchestrates glycaemic responses to stress
Nature. 2025 https://doi.org/10.1038/s41586-025-09420-1
