This week’s journal club discusses a landmark first-in-human study published in Cell (2024) investigating autologous transplantation of chemically induced pluripotent stem cell-derived islets (CiPSC-islets) for type 1 diabetes (T1D). A 25-year-old woman with long-standing, poorly controlled T1D and a history of failed pancreas transplantation received patient-specific CiPSC-islets generated from adipose-derived stromal cells through chemical reprogramming. The islets were transplanted beneath the abdominal anterior rectus sheath, an extrahepatic site optimized for survival and monitoring.
Clinical and metabolic outcomes were evaluated using exogenous insulin requirement, HbA1c, continuous glucose monitoring (CGM), fasting blood glucose, and oral glucose tolerance testing. Functional engraftment was assessed by plasma C-peptide levels, β-cell responsiveness, and BETA-2 scoring, while safety was monitored through MRI imaging, tumor marker assays, and adverse event reporting.
The patient achieved insulin independence by day 75 post-transplantation and maintained it for one year. HbA1c decreased from 7.6% to ~5% and remained in the non-diabetic range, while CGM revealed a time-in-target glycemic range >98% compared to 43% pre-transplantation. Fasting C-peptide rose from undetectable levels to 400–700 pmol/L, with strong glucose-stimulated responses, confirming functional β-cell restoration. Imaging and biomarker analyses showed no evidence of teratoma, graft overgrowth, or rejection, supporting the safety of the approach.
In conclusion, this first patient outcome demonstrates the feasibility and therapeutic promise of CiPSC-islet autologous transplantation in achieving durable, insulin-independent glycemic control without major complications. While limited to a single case with ongoing immunosuppression, these findings provide a strong rationale for further clinical studies exploring CiPSC-based regenerative medicine for diabetes.
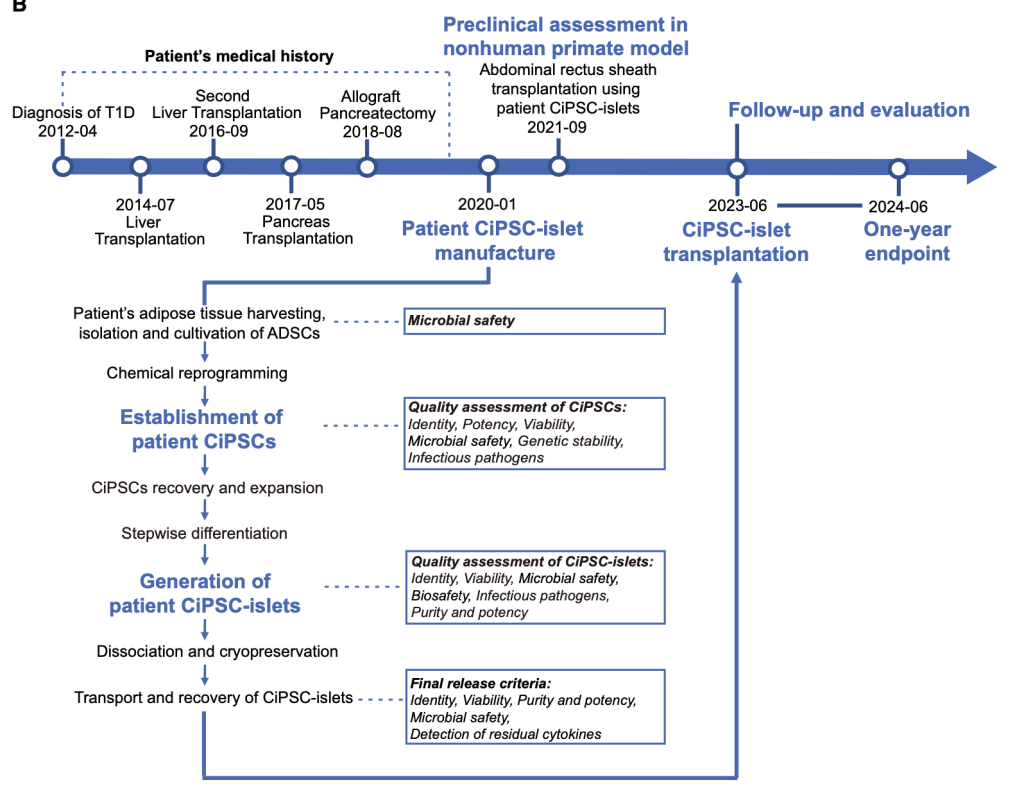
Figure from Wang et al. Timeline showing the patient’s medical history, key time points of this study, as well as the workflow and quality controls during the manufacturing process of the patient-derived CiPSC-islets.
Highlights:
- Patient-derived islets were generated with chemically induced pluripotent stem cells
- Transplantation of these islets to an abdominal site led to engraftment in one patient
- Exogenous insulin-independent glycemic control was restored in the patient
- All safety and efficacy clinical endpoints were met at 1-year follow-up of the patient
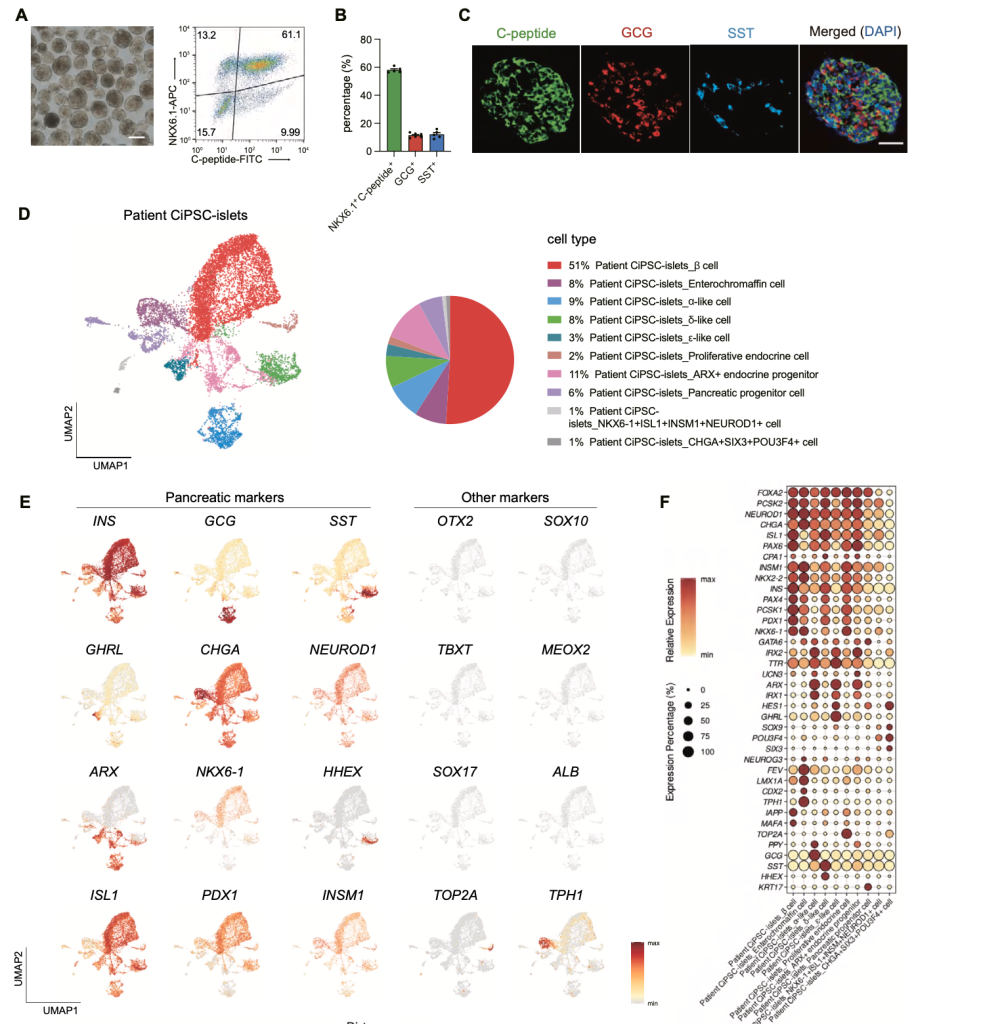
Figure from Wang et al. Characterization of patient CiPSC-islets
Journal Club: continue your reading here:

1 comment for “Journal Club: Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient”