Rethinking the Overshadowed
Glucose homeostasis in the human body is maintained through a delicate balance between glucose production, uptake, and storage, primarily regulated by endocrine hormones secreted from the pancreas. While insulin from β cells and glucagon from α cells are well-established regulators of blood glucose homeostasis, the contribution of δ cells has been established as a paracrine regulator of insulin and glucagon secretion from β and α cells. This regulatory role makes δ cells a checkpoint of overall glucose homeostasis in the hierarchy. Few studies have been conducted on the demultiplexing role of δ cells in the context of pancreatic regulation until recently.
Recent research, particularly the study published in Nature Metabolism by Huang et al. (2024) has established somatostatin as a determinant of the glycaemic set point.
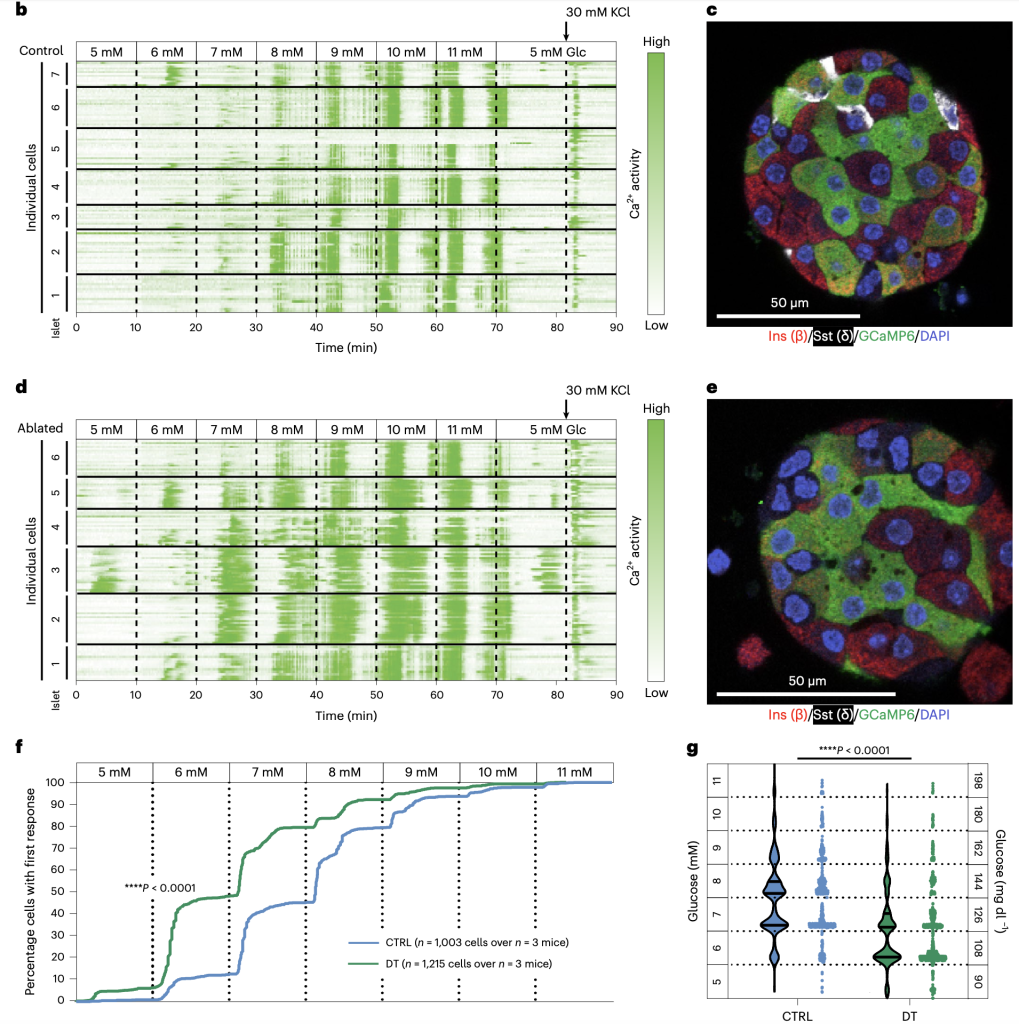
The study systematically examined the role of pancreatic δ cells in glucose homeostasis using three distinct mouse models, which allowed for the targeted removal of SST signaling. These findings were corroborated using in vivo and ex vivo approaches, including glucose tolerance tests, plasma insulin measurements, and calcium imaging of pancreatic islets. The absence of SST in mice resulted in a sustained decrease in glycaemia. Diphtheria toxin (DT) mediated the ablation of δ cells in adult mice, resulting in a decrease in glycemia as well as lowering the glucose threshold. Transplanting SST-deficient islets into mice re-established a glycaemic set point corresponding to the donor islets, reinforcing the conclusion that SST acts locally within the islet to regulate glucose homeostasis. DT-mediated α cell ablation showing no impact on glycemic set point, glucose tolerance, or plasma insulin level further established SST signaling as the key regulator. Transplantation of islets and DT ablation of δ cells in STZ-treated hyperglycemic mice re-established a glycaemic set point corresponding to the donor islets, reinforcing the conclusion. In order to confirm the glycaemic homeostatic impact is primarily due to pancreatic δ cell secreted somatostatin, the study also showed somatostatin recovery in the stomach, intestine, and brain post-ablation.
The implication of this research extends toward clinical opportunities on the horizon. Understanding its impact in disease-based models will establish somatostatin as a strong candidate for novel pharmacological targets. Diabetes, being a complex disease involving different causalities for hyperglycemia, would be a realistic tool to characterize the extent of somatostatin impact.
To conclude, the research by Huang et al. (2024) highlights somatostatin’s role in glucose homeostasis. Their findings show that disrupting SST signaling leads to enhanced insulin secretion and a lower glycemic set point, offering new insights into metabolic regulation. Addressing the existing challenges and expanding studies into human-relevant models will be crucial in translating these insights into clinical applications. Future research should focus on exploring SST’s therapeutic potential and its role in diabetes management.
Continue your reading here:
Huang JL, Pourhosseinzadeh MS, Lee S, Krämer N, Guillen JV, Cinque NH, Aniceto P, Momen AT, Koike S, Huising MO. Paracrine signalling by pancreatic δ cells determines the glycaemic set point in mice. Nat Metab. 2024 Jan;6(1):61-77. doi: 10.1038/s42255-023-00944-2.